The CMF OL1000 Bone Growth Stimulators are portable, battery-powered medical devices indicated for use in the noninvasive treatment of an established nonunion fracture acquired secondary to trauma, excluding all vertebrae and flat bones. They come in a single coil or dual coil format. The single coil version comes in 4 different sizes. The size and coil configuration depends on the fracture site.
Key Features of the CMF OL1000 Bone Growth Stimulator
- Lightweight and comfortable
- Easy-to-use & Noninvasive
- Requires simple, one-button operation
- Device is worn for 30 minutes per day
- Can be used with internal or external fixation or over a cast
- Clinical studies reported success rates as high as 89%
How does the CMF OL1000 Bone Growth Stimulator Actually Work?
The CMF OL 1000 non-invasive bone growth stimulator produces very low energy combined static and dynamic magnetic fields on the order of the earth’s magnetic field. The device has a push-button that starts the treatment and audible tones to notify the patient that a treatment has started or has ended. A Liquid Crystal Display (LCD) is used to display the device status, e.g., treatment record, daily treatment time countdown.
There are Three major components to the device:
- An Electronic Control Module
- A Transducer Coil
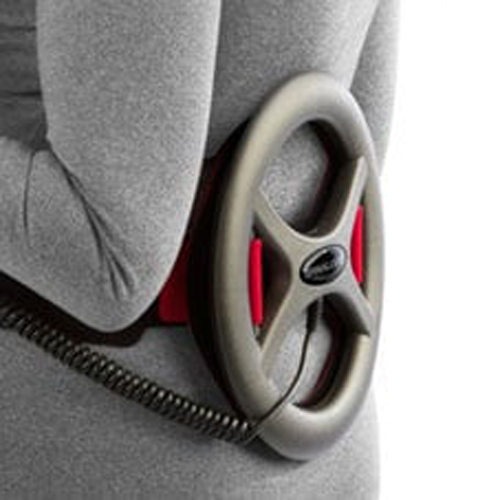
The Electronic Control Module includes a signal generator that produces an electrical signal which is transmitted to the treatment transducer. The transducer coil converts the electrical signal into a magnetic field. The coil is placed facing the spine so that the magnetic field is directed at the fusion site. The waist belt is designed to secure this coil relative to the patient’s fusion site during treatment.
The CMF OL1000 bone growth stimulator provides treatment by exposing the fusion site to a low energy magnetic field, which is undetectable during treatment. The patient will typically have no sensation related to the treatment.
CMF OL1000 Device Measurement Guide
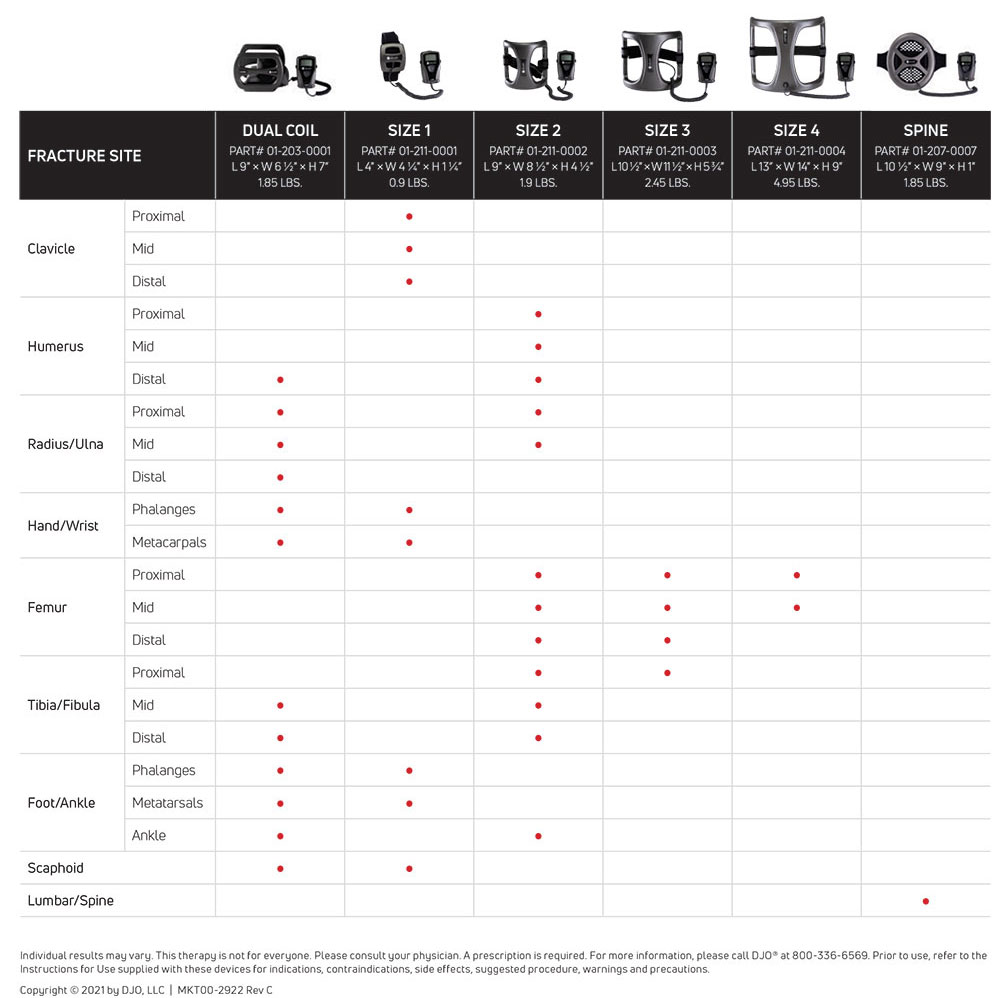
Product videos for the DJO CMF OL1000 Bone Growth Stimulator
CMF Signal Science
How to Apply Your CMF OL1000 Single Coil Size 1 Bone Growth Stimulator
How to Apply Your CMF OL1000 Single Coil Size 2,3,4 Bone Growth Stimulator
CMF OL1000 Bone Growth Stimulator – Using Your Control Unit
CMF OL1000 Dual Coil Bone Growth Stimulator – How To Apply
Who can benefit from using a Bone Growth Stimulator?
Let your doctor know about any health condition which could affect how your bones heal when consulting with your doctor about a fracture. There are pre-existing genetic conditions, as well as lifestyle choices, that can negatively impact how long your bones take to heal or the likelihood of successful new bone formation.
The following medical conditions are generally considered to be medically necessary reasons for a bone growth stimulator prescription:
- Previous non-union bone fracture
- Failed fusion of a joint
- Congenital pseudarthrosis
- Chemotherapy, diabetes, obesity, osteoporosis, renal disease, tobacco use, steroid use
Indications For Using the CMF OL1000 Bone Growth Stimulator
- Noninvasive treatment of an established nonunion acquired secondary to trauma, excluding vertebrae and flat bones.
- A nonunion is considered to be established when the fracture site shows no visibly progressive signs of healing.
Contraindications to using the CMF OL1000 Bone Growth Stimulator
Use of this device is contraindicated in individuals having a synovial pseudarthrosis. Demand-type pacemaker or implantable cardioverter defibrillator (ICD) operation may be adversely affected by exposure to magnetic fields. Physicians should not prescribe CMF 0L1000 for applications that may place the treatment transducers in close proximity to the pacemaker. Further screening by the attending cardiologist is recommended (such as with an electrocardiogram).
CMF 0L1000 should not be used in the presence of external or internal fixation devices that are constructed from magnetic materials. (NOTE: Almost all fracture fixation devices implanted today are made from nonmagnetic materials.)
Additional patient warnings, precautions, and adverse events can be referenced in the CMF OL1000 Instruction Manual.
Warnings
The safety and effectiveness of the use of this device on individuals lacking skeletal maturity have not been established. Animal studies conducted to date do not suggest any long-term significant adverse effects from use of this device. However, long-term effects in humans are unknown. Teratological studies have not been performed with this device. The safety of use of this device during pregnancy or nursing in humans has not been established.
Precautions
Weight bearing is not advised in the presence of extreme motion at the nonunion site. In the presence of a malaligned nonunion, careful consideration of the use of this device must be undertaken on an individual basis, as treatment with this device is not intended to alter or affect the degree of malalignment.
The safety and effectiveness of the use of this device on individuals with nonunion secondary to, or in conjunction with, a pathological condition have not been established.
This device should not be used if there are mental or physical conditions that preclude patient compliance with the physician and device instructions. When conditions of atrophy are present or when fractures have remained unhealed for long periods of time, there may be less successful results.
Adverse Effects
No known significant adverse effects have resulted from the use of this device. Clinical studies, animal studies, and tissue culture experiments conducted with the OL1000, which has the same treatment signal as the OL1000 SC1, have not indicated any evidence of significant adverse effects.
Caution
Federal law (U.S.A. and Canada) restricts this device to sale, distribution or use by or on the order of a physician.
Recommended Treatment Frequency of the CMF OL1000 Bone Growth Stimulator
Prescribed treatment time is typically 30 minutes per day. The CMF OL1000 device is designed to deliver daily treatments for up to 270 days. Your doctor will determine the appropriate therapy duration for your condition.
Documents PDFs – CMF OL1000 Bone Growth Stimulator
![]() CMF OL1000 CMF OL1000 Brochure.pdf
CMF OL1000 CMF OL1000 Brochure.pdf
![]() CMF OL1000 Clinical Research Paper
CMF OL1000 Clinical Research Paper
![]() CMF OL1000 Published Pre-Clinical Paper
CMF OL1000 Published Pre-Clinical Paper
![]() CMF Bone Growth Stimulation Scientific Data Sheet
CMF Bone Growth Stimulation Scientific Data Sheet
References for the CMF OL1000 Bone Growth Stimulator
- Dates represent first FDA approval of technology – technology may have been approved for other products at later dates. ⁵McLeod, K.J., Rubin, C.T., The Effect of Low Frequency Electrical Fields on Osteogenesis. J. Bone Joint Surg., 74A: 920 – 929, 1992.
- Ryaby, J.T., et al., The Role of Insulin-like Growth Factor in Magnetic Field Regulation of Bone Formation, Bioelectrochemistry and Bioenergetics, 35: 87-91, 1994.
- Linovitz R, Pathria M, Bernhardt M, et al. Combined Magnetic Fields Accelerate and Increase Spine Fusion: A Double-Blind, Randomized, Placebo Controlled Study, Spine. 2002 July; 27(13):1383-1388. ⁹NASS Coverage Policy Recommendations for Electrical Bone Growth Stimulators, 2016.












 Ask A Question
Ask A Question
 Tax Exempt Shopping
Tax Exempt Shopping
 Get Clinical Pricing
Get Clinical Pricing
 Affiliate Program
Affiliate Program
 Retail Stores
Retail Stores
 Health Hub
Health Hub
 Shipping Policy
Shipping Policy
 Returns/Exchanges
Returns/Exchanges
 About Us
About Us
 Contact Us
Contact Us




 BIOFLEX P120 Light Therapy System
BIOFLEX P120 Light Therapy System



















































Reviews
There are no reviews yet